Non-24: It’s about timing
Non-24 is marked by a continuous shift of the sleep-wake cycle1
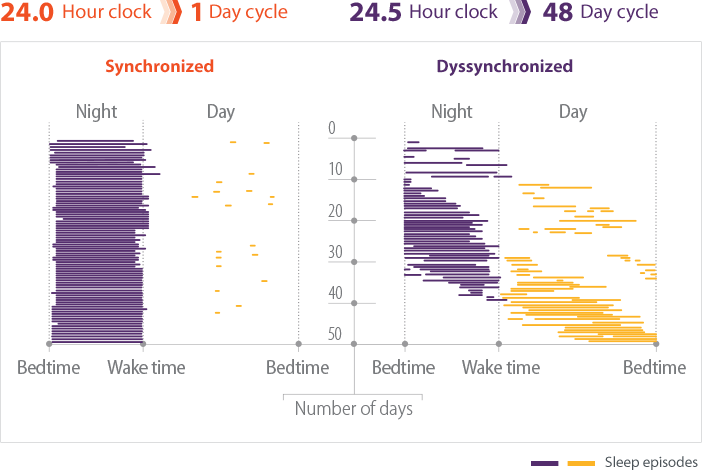
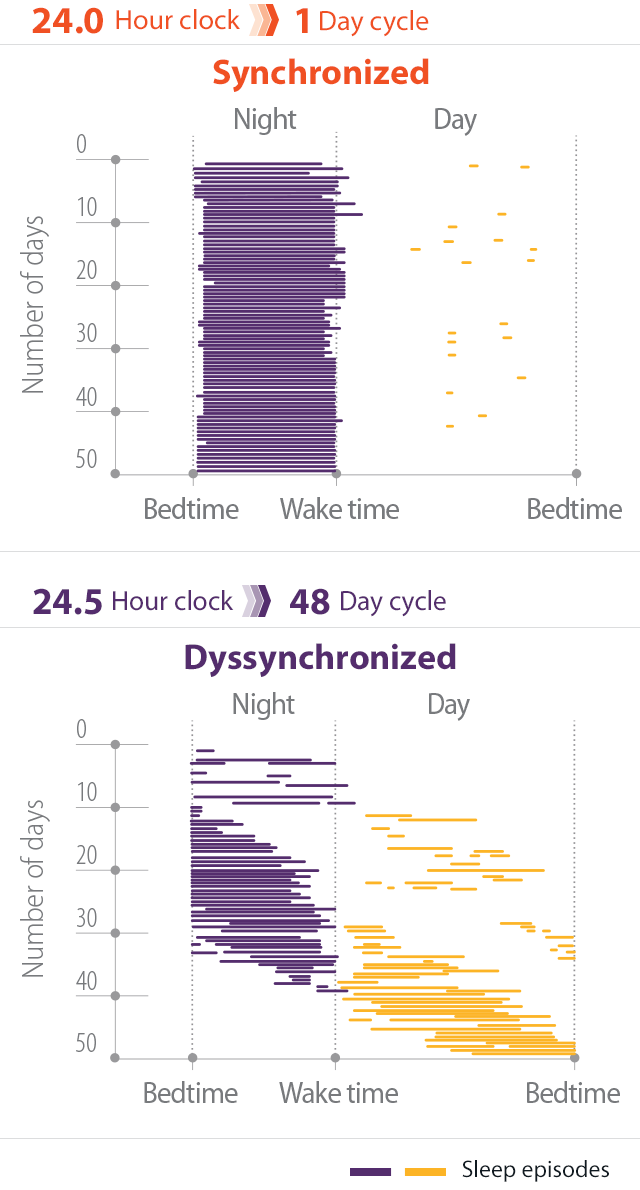
- In individuals without Non-24, light is the most potent mechanism for aligning the natural cycle of sleep and wakefulness with the 24-hour day2,3
- Patients with Non-24 experience shifting sleep-wake cycles, marked by periods of poor sleep, broken up by periods of symptomic relief when the sleep-wake cycle is aligned with the 24-hour day4
- These periods may last for weeks or months5
References: 1. Dressman MA, et al. Poster presented at: 26th Annual Meeting of the Associated Professional Sleep Societies, LLC; June 10, 2012; Boston, MA. Poster 49. 2. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. 3. Dibner C, et al. Annu Rev Physiol. 2010;72:517-549. 4. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. 5. Lockley SW, et al. In: Kushida C, ed. The Encyclopedia of Sleep. Vol 3. Waltham, MA: Elsevier; 2013:34-40.
![HETLIOZ[R] (tasimelteon) capsules 20mg](/assets/header/header-logo-ed4a0eafea0a6d3ceec1cd9c4d717dea66814d23c9da61298b3f9181ff9546a6.jpg)


