Role of light perception in Non-24
In sighted individuals: Daily exposure to the light-dark cycle synchronizes the circadian clock with the 24-hour day.1,2
Ability to
perceive light
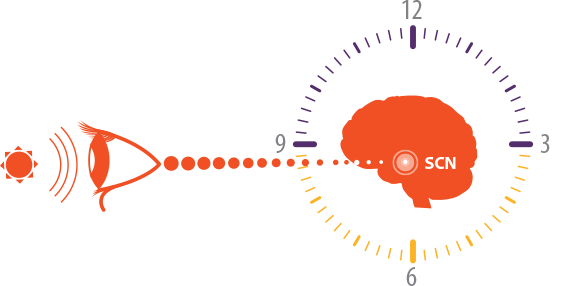
SCN, suprachiasmatic nucleus.
The SCN controls daily circadian rhythms, including the production of melatonin and cortisol, as well as body temperature. Lack of synchronization of the SCN results in the misalignment of these circadian rhythms, including the sleep-wake cycle, which can differ between individuals.3
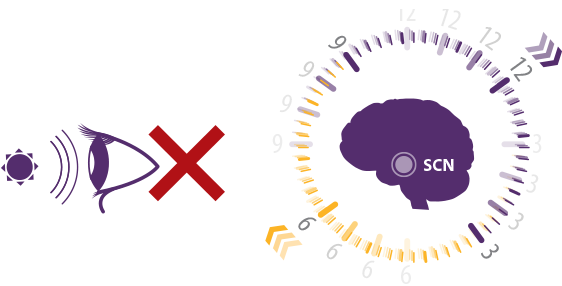
In most people who are totally blind: Without light perception, sleep-wake cycles shift, often becoming dyssynchronized with the 24-hour day.1
Inability to
perceive light
References: 1. Lockley SW, et al. In: Kushida C, ed. The Encyclopedia of Sleep. Vol 3. Waltham, MA: Elsevier; 2013:34-40. 2. American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. 3. Sack RL, et al. J Clin Endocrinol Metab. 1992;75(1):127-134.
![HETLIOZ[R] (tasimelteon) capsules 20mg](/assets/header/header-logo-ed4a0eafea0a6d3ceec1cd9c4d717dea66814d23c9da61298b3f9181ff9546a6.jpg)


